Distinction of trans–cis photoisomers with comparable optical properties in multiple-state photochromic systems – examining a molecule with three azobenzenes via in situ irradiation NMR spectroscopy†
Abstract
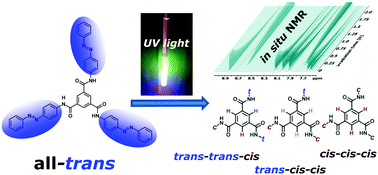
Photochromic compounds like azobenzenes are widely used for the production of stimuli responsive materials. To analyse cascaded azobenzene switching inside a benzene-tricarboxamide (BTA) with three azobenzene moieties in a site-specific fashion, we used in situ irradiation NMR spectroscopy. Four photoisomers can be distinguished by their chemical shifts. Analysis of 1H, 13C and 15N shifts reveals that the configuration of one sidechain has an influence on the chemical shifts of both the other sidechains. Interconversion kinetics upon irradiation with ultraviolet (UV) light as well as molar fractions in photostationary states (PSS) were examined. Analysis of thermal fading of the different photoisomers into the ground state shows that thermal relaxation rates of all three azobenzene moieties behave as if they were independent of each other.


 Please wait while we load your content...
Please wait while we load your content...