Lewis acid catalysis: catalytic hydroboration of alkynes initiated by Piers' borane†
Abstract
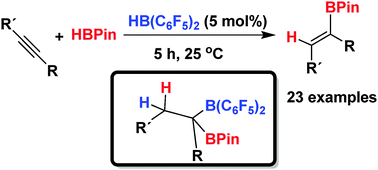
Terminal and internal alkynes are efficiently hydroborated to (E)-alkenyl pinacol boronic esters with excellent yields and selectivities using a Lewis acid catalyst. In the case of Piers' borane (HB(C6F5)2) the borane acts as a pre-catalyst generating dissymmetrically gem-diborylated species of the form RCH2CR′(Bpin)(B(C6F5)2) which are the active catalysts.

 Please wait while we load your content...
Please wait while we load your content...