Direct amidation of unprotected amino acids using B(OCH2CF3)3†
Abstract
A commercially available borate ester, B(OCH2CF3)3, can be used to achieve protecting-group free direct amidation of α-amino acids with a range of amines in cyclopentyl methyl ether. The method can be applied to the synthesis of medicinally relevant compounds, and can be scaled up to obtain gram quantities of products.
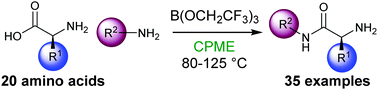


 Please wait while we load your content...
Please wait while we load your content...