A copper catalyzed azidation and peroxidation of β-naphthols via an oxidative dearomatization strategy†
Abstract
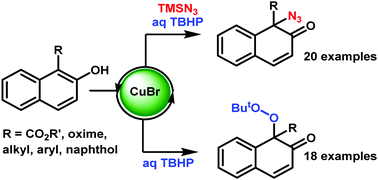
Dearomatizative azidation and peroxidation of β-naphthols have been explored using copper bromide as a catalyst. These reactions lead to highly valuable naphthalenone derivatives such as quaternary azide derivatives and quaternary peroxide derivatives in good yields. This method paves an excellent way for synthesizing azides and peroxides which serve as masked surrogates for their corresponding amines and alcohols. Using this method, optically pure binol derivatives were transformed to their corresponding chiral naphthalenones in excellent yields with moderate enantioselectivity.

 Please wait while we load your content...
Please wait while we load your content...