Protonated hexaphyrin–cyclodextrin hybrids: molecular recognition tuned by a kinetic-to-thermodynamic topological adaptation†
Abstract
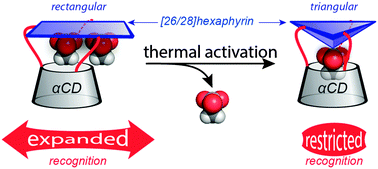
Protonation study of [26/28]hexaphyrin-capped cyclodextrins revealed a temperature controlled conformational transition of the cap. The hexaphyrin undergoes a rectangular-to-triangular shape-shifting which strongly modifies the shape of the confined environment featured by the hybrids, and ultimately affects the encapsulation of the counterions. It provides an attractive access to innovative allosteric host–guest systems.

 Please wait while we load your content...
Please wait while we load your content...