Peroxodisulfate-mediated selenoamination of alkenes yielding amidoselenide-containing sulfamides and azoles†
Abstract
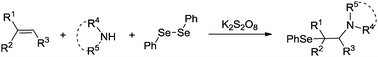
A new protocol for C–Se and C–N bond formation by the direct difunctionalization of alkenes is reported. The protocol is operationally simple, has a wide substrate scope, and uses readily available amino sources. This reaction represents a significant addition to the limited number of intermolecular selenide difunctionalization reactions of alkenes and would find practical application in the synthesis of nitrogen- and selenium-containing molecules.


 Please wait while we load your content...
Please wait while we load your content...