Gold-catalysed facile access to indene scaffolds via sequential C–H functionalization and 5-endo-dig carbocyclization†
Abstract
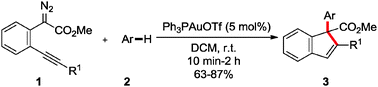
A concise synthesis of functionalized indene derivatives via the gold(I)-catalysed cascade C–H functionalization/conia-ene type reaction of electron-rich aromatics with o-alkynylaryl α-diazoesters has been developed. In this transformation, the gold catalyst not only catalysed the formation of the zwitterionic intermediate via intermolecular C–H functionalization but promoted the subsequent intramolecular 5-endo-dig cyclization via activation of alkynes. The reaction is characterized by high chemo- and site-selectivity, readily available starting materials, nice functional-group tolerance and mild reaction conditions.

 Please wait while we load your content...
Please wait while we load your content...