Simple magnesium catalyst mediated γ-butyrolactams in desymmetrization of meso-aziridines†
Abstract
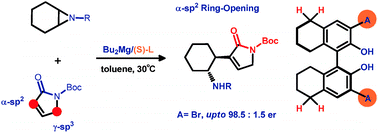
The first α-sp2-carbon of carbonyl compounds attacked catalytic desymmetrization reaction of aziridines is disclosed. A simple in situ generated magnesium catalyst from 3,3′-bromine-8H-BINOL and dibutylmagnesium was employed. It is interesting that the bromine atom on the chiral ligand plays a key role in introducing a high level of enantioselectivities and high reaction efficiency. Both enantiomers of the ring-opening product could be smoothly obtained by using (R)- and (S)-L7.

 Please wait while we load your content...
Please wait while we load your content...