Identification of genes encoding squalestatin S1 biosynthesis and in vitro production of new squalestatin analogues†
Abstract
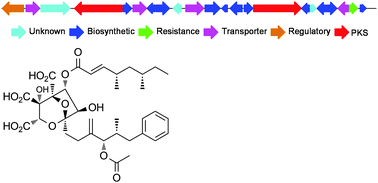
A gene cluster responsible for the biosynthesis of squalestatin S1 (SQS1, 1) was identified by full genome sequencing of two SQS1-producing ascomycetes: Phoma sp. C2932 and unidentified fungus MF5453. A transformation protocol was established and a subsequent knockout of one PKS gene from the cluster led to loss of SQS1 production and enhanced concentration of an SQS1 precursor. An acyltransferase gene from the cluster was expressed in E. coli and the expressed protein MfM4 shown to be responsible for loading acyl groups from CoA onto the squalestatin core as the final step of biosynthesis. MfM4 appears to have a broad substrate selectivity for its acyl CoA substrate, allowing the in vitro synthesis of novel squalestatins.

 Please wait while we load your content...
Please wait while we load your content...