Ca(BH4)2–Mg2NiH4: on the pathway to a Ca(BH4)2 system with a reversible hydrogen cycle
Abstract
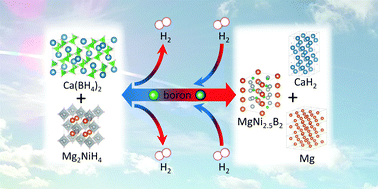
The Ca(BH4)2–Mg2NiH4 system presented here is, to the best of our knowledge, the first described Ca(BH4)2-based hydride composite that reversibly transfers boron from the Ca-based compound(s) to the reaction partner. The ternary boride MgNi2.5B2 is formed upon dehydrogenation and the formation of Ca(BH4)2 upon rehydrogenation is confirmed.


 Please wait while we load your content...
Please wait while we load your content...