Towards an all-copper redox flow battery based on a copper-containing ionic liquid†
Abstract
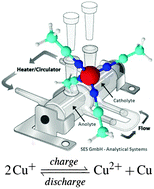
The first redox flow battery (RFB), based on the all-copper liquid metal salt [Cu(MeCN)4][Tf2N], is presented. Liquid metal salts (LMS) are a new type of ionic liquid that functions both as solvent and electrolyte. Non-aqueous electrolytes have advantages over water-based solutions, such as a larger electrochemical window and large thermal stability. The proof-of-concept is given that LMSs can be used as the electrolyte in RFBs. The main advantage of [Cu(MeCN)4][Tf2N] is the high copper concentration, and thus high charge and energy densities of 300 kC l−1 and 75 W h l−1 respectively, since the copper(I) ions form an integral part of the electrolyte. A Coulombic efficiency up to 85% could be reached.

 Please wait while we load your content...
Please wait while we load your content...