Metal toxicity and the p53 protein: an intimate relationship
Abstract
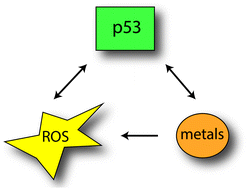
Many metals are pivotal for the function of enzymes and proteins, but in excess can result in the formation of free radicals (ROS) that can cause damage to DNA. p53 is a tumour suppressor protein and transcription factor that can react to DNA damage to either facilitate a repair response or when the damage is too severe to initiate apoptosis and cell death. ROS can therefore activate p53, but the p53 protein itself is susceptible to oxidative changes, resulting in unfolding of the p53 protein and therefore loss of activity. In order to be active, p53 needs to bind zinc while other metals such as copper can displace zinc leading to p53 unfolding. Metal exposure can therefore result in the activation or inactivation of p53 directly and indirectly through ROS, all dependent on the type of metal and the severity of the exposure. Conversely, p53 itself can impact on ROS and affect the expression of important proteins in metal cell biology. In the current review, we have summarized the relationship between p53, ROS and metal toxicity.
- This article is part of the themed collection: New Talents

 Please wait while we load your content...
Please wait while we load your content...