Effect of the cyano group on solid state photophysical behavior of tetraphenylethene substituted benzothiadiazoles†
Abstract
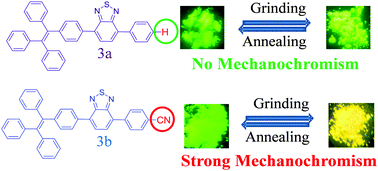
Two unsymmetrical tetraphenylethene (TPE) substituted Donor–Acceptor (D–A) benzothiadiazoles (BTDs) 3a, and 3b were designed and synthesized by the Suzuki cross-coupling reaction. The design strategy was opted to maintain the donor (TPE) fragment constant and the acceptor strength of BTD was modulated by using phenyl and cyanophenyl units. Their solvatochromism, aggregation induced emission (AIE) and mechanochromic properties were investigated. The BTDs 3a, and 3b exhibit strong solvatochromic and AIE behavior. The cyano-group containing BTD 3b exhibits reversible mechanochromic behavior with high color contrast between green and yellow, whereas 3a do not show mechanochromism. The solid state absorption and emission properties of BTDs 3a, and 3b show different behavior in their pristine and ground form. The powder XRD study shows a reversible morphological change between the crystalline and amorphous phase upon grinding.

 Please wait while we load your content...
Please wait while we load your content...