The effect of regioisomerism on the solid-state fluorescence of bis(piperidyl)anthracenes: structurally simple but bright AIE luminogens†
Abstract
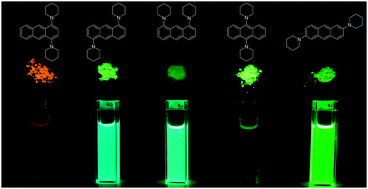
A series of regioisomers of piperidylanthracenes (PA) and bis(piperidyl)anthracenes (BPA) were synthesized and their photophysical properties examined in solution, suspension, and the solid state. Aggregation-induced emission (AIE) was observed only for 1,4-BPA and 9,10-BPA, in which two piperidyl groups are substituted at the anthracene moiety in the para-position with respect to each other. Compared to previously reported AIE luminogens, these easily obtainable para-substituted BPAs, exhibited several unique and beneficial features, such as simple structures, bright solid-state fluorescence (Φfl = 0.49 and 0.86 for 1,4-BPA and 9,10-BPA, respectively), tunable fluorescence emission, and large Stokes shifts. Results from diffuse-reflectance and fluorescence lifetime measurements demonstrated that PAs and BPAs intrinsically possess an efficient non-radiative transition pathway in the solid state, and that 1,4-BPA and 9,10-BPA may overcome this pathway. The X-ray crystallographic analysis of 1,4-BPA revealed that undesirable interchromophoric interactions can be minimised, while TD-DFT calculations suggested that the enhanced Stokes shift of 1,4-BPA arises from severe electronic repulsion between neighbouring piperidyl moieties, which presumably results in the absence of self-absorption.
- This article is part of the themed collection: 20th Anniversary of Aggregation-Induced Emission

 Please wait while we load your content...
Please wait while we load your content...