Development of ion conducting polymer gel electrolyte membranes based on polymer PVdF-HFP, BMIMTFSI ionic liquid and the Li-salt with improved electrical, thermal and structural properties†
Abstract
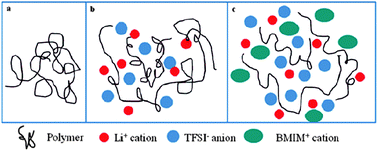
Ion conducting polymer gel electrolyte membranes based on polymer poly(vinylidene fluoride-co-hexafluoropropylene) PVdF-HFP, ionic liquid, 1-butyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide BMIMTFSI with and without the Li-salt (having the same anion i.e. the TFSI− anion) have been synthesized. Prepared membranes have been characterized by scanning electron microscopy, X-ray diffraction, Fourier transform infrared (FTIR), differential scanning calorimetry, thermogravimetric analysis (TGA) and complex impedance spectroscopic techniques. Incorporation of IL in the polymer PVdF-HFP/polymer electrolyte (i.e. PVdF-HFP + 20 wt% LiTFSI) changes different physicochemical properties such as melting temperature (Tm), glass transition temperature (Tg), thermal stability, degree of crystallinity (Xc), and ionic transport behaviour of these materials. The ionic conductivity of polymeric gel electrolyte membranes has been found to increase with increasing concentration of IL and attains a maximum value of 2 × 10−3 S cm−1 at 30 °C and ∼3 × 10−2 S cm−1 at 130 °C. A high total ionic transference number >0.99 and the cationic transference number (tLi+) ∼ 0.22 with a wider electrochemical window (ECW) ∼ 4.0–5.0 V for the polymer gel electrolyte membrane containing higher loading of IL (∼70 wt% of IL) have been obtained. Temperature dependent ionic conductivity obeys Arrhenius type thermally activated behaviour.

 Please wait while we load your content...
Please wait while we load your content...