Control over Fe3+ speciation in colloidal ZnO nanocrystals†
Abstract
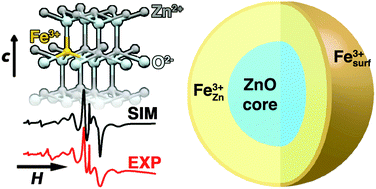
The incorporation of potentially redox active dopant ions holds much promise for applications in catalysis and energy. Here we report the room-temperature synthesis of colloidal Fe-doped ZnO nanocrystals. By combining detailed dopant-specific spectroscopy with known single crystal data we are able to elucidate the locations of paramagnetic Fe3+ ions in the colloidal ZnO nanocrystals. Electron paramagnetic resonance (EPR) spectra of 0.15–2.0% Fe-doped ZnO nanocrystals are consistent with the Fe dopants occupying both pseudo-tetrahedral (substitutional at the Zn-site) and pseudo-octahedral (surface and interstitial) coordination environments. The evolution of the spectra as a function of ZnO growth time allow us to provide additional mechanistic insight into the formation of doped colloidal ZnO nanocrystals using a simple room temperature synthetic method. We also demonstrate control over the speciation of the Fe dopants in colloidal ZnO nanocrystals by changing the growth and/or surface-ligand treatment times.

 Please wait while we load your content...
Please wait while we load your content...