Improved synthesis and photovoltaic performance of donor–acceptor copolymers based on dibenzothiophene-cored ladder-type heptacyclic units†
Abstract
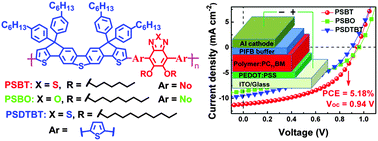
We have developed a facile synthetic route to a ladder-type donor unit (SDCT) wherein two outer thiophene subunits are covalently fastened to the central dibenzothiophene core through two sp3-hybridized bridging carbons. An innovative transformation from an aryl ketone group to an aryl ester group was applied to construct the ladder-type molecular skeleton, and the overall synthetic yield toward the donor unit has been significantly improved by choosing aryl side chains instead of aliphatic ones to avoid competing dehydration reactions. To reveal the effects of π-spacers and heteroatom substituents, three donor–acceptor (D–A) copolymers containing SDCT and acceptor units of 2,1,3-benzothiadiazole (BT), 2,1,3-benzoxadiazole (BO), or 4,7-bis(2-thienyl)-2,1,3-benzothiadiazole (DTBT) were synthesized, characterized and used for polymer solar cells (PSCs). All polymers exhibit blue-shifted absorption spectra and deeper-lying HOMO energy levels compared to the previous carbazole-based skeleton analogues. In comparison with its analogous polymer with the same π-conjugated backbone, the polymer with alkoxy-substituted BT as the acceptor unit (PSBT) shows an order of magnitude higher OFET mobility (1.8 × 10−4versus 1.25 × 10−5 cm2 V−1 s−1). An optimal device based on PSBT : PC71BM (1/3 in wt%) delivers a respectable PCE of 5.18% and a high Voc of 0.94 V, all of which are superior to those of the carbazole-based analogue (PCE = 3.7%, Voc = 0.80 V) and greatly surpass the values of a previously reported dibenzothiophene-based polymer (PCE = 0.76%, Voc = 0.64 V). These results demonstrate that SDCT is a promising building block for constructing photovoltaic polymers and the synthetic strategy developed herein can be used to prepare other dibenzothiophene-cored ladder-type heptacyclic units.

 Please wait while we load your content...
Please wait while we load your content...