Aqueous-based synthesis of gallium tungsten oxide thin film dielectrics†
Abstract
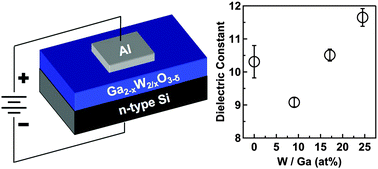
An aqueous-based approach to the deposition of gallium tungsten oxide thin film dielectrics was developed where the metal content (Ga2−xWx/2O3−δ, x = 0.31–0.66) can be controlled by the initial precursor concentrations, while the oxygen deficiency (δ) can be controlled by post annealing. The spin-coated precursor converts to an amorphous oxide thin film with uniform distribution of metal cations after heating to 400 °C. The Ga2−xWx/2O3−δ band gap decreased with increasing W content from 4.9 eV for the undoped film (Ga2O3) to 4.6 eV for the film with the highest W content (Ga1.34W0.33O3−δ). The electronic properties of the dielectric films significantly improved with incorporation of W, where a decrease in leakage current and an increase in breakdown field strength from 2.9 MV cm−1 (Ga2O3) to 5.2 MV cm−1 (Ga1.34W0.33O3−δ) was observed. Prior studies have suggested that Ga2−xWx/2O3−δ thin films can form dense low-k dielectric materials, where the relative amount of W in the film can significantly modify the dielectric constant. However our studies found no significant decrease in the dielectric constant with W doping of Ga2O3. We found that annealing the films >400 °C in air resulted in the formation of sub-stoichiometric WOx and metallic W throughout the films which may complicate dielectric measurements.

 Please wait while we load your content...
Please wait while we load your content...