A bridged low band gap A–D–A quaterthiophene as efficient donor for organic solar cells†
Abstract
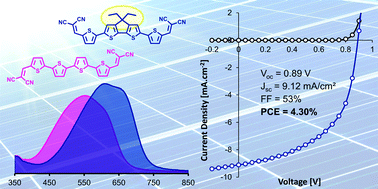
α,ω-Bis(dicyanovinyl)quaterthiophene 1 with a median 4,4-diethyl-4H-cyclopenta[2,1-b:3,4-b′]dithiophene has been synthesized. UV-Vis absorption data show that the covalent bridging of the inner 2,2′-bithiophene leads to a significant reduction of the HOMO–LUMO gap essentially due to an increase of the HOMO level as confirmed by electrochemical and theoretical results. X-ray diffraction analysis of a single crystal of 1 shows that except for the out-of-plane ethyl groups, the conjugated system displays a quasi-planar geometry while the molecular packing exhibits strong π-stacking interactions and multiple short intermolecular contacts. Quaterthiophene 1 has been used as active donor material in organic solar cells of various architectures including bi-layer planar hetero-junctions and hybrid co-evaporated bulk hetero-junctions with C60 as electron acceptor material. A maximum conversion efficiency of 4.30% is obtained with a hybrid co-evaporated device. These results are discussed in terms of structure–properties relationships with reference to the open-chain parent α,ω-bis(dicyanovinyl)quaterthiophene 2.

 Please wait while we load your content...
Please wait while we load your content...