Nanostructure of wet-chemically prepared, polymer-stabilized silver–gold nanoalloys (6 nm) over the entire composition range
Abstract
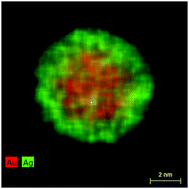
Bimetallic silver–gold nanoparticles were prepared by co-reduction using citrate and tannic acid in aqueous solution and colloidally stabilized with poly(N-vinylpyrrolidone) (PVP). The full composition range of silver : gold from 0 : 100 to 100 : 0 (n : n) was prepared with steps of 10 mol%. The nanoparticles were spherical, monodispersed, and had a diameter of ∼6 nm, except for Ag : Au 90 : 10 nanoparticles and pure Ag nanoparticles which were slightly larger. The size of the nanoalloys was determined by differential centrifugal sedimentation (DCS) and transmission electron microscopy (TEM). By means of X-ray powder diffraction (XRD) together with Rietveld refinement, precise lattice parameters, crystallite size and microstrain were determined. Scanning transmission electron microscopy (STEM) combined with energy-dispersive X-ray spectroscopy (EDX) and electron energy loss spectroscopy (EELS) showed that the particles consisted of a gold-rich core and a silver-rich shell. XRD and DCS indicated that the nanoparticles were not twinned, except for pure Ag and Ag : Au 90 : 10, although different domains were visible in the TEM. A remarkable negative deviation from Vegard's linear rule of alloy mixtures was observed (isotropic contraction of the cubic unit cell with a minimum at a 50 : 50 composition). This effect was also found for Ag:Au bulk alloys, but it was much more pronounced for the nanoalloys. Notably, it was much less pronounced for pure silver and gold nanoparticles. The microstrain was increased along with the contraction of the unit cell with a broad maximum at a 50 : 50 composition. The synthesis is based on aqueous solvents and can be easily scaled up to a yield of several mg of a well dispersed nanoalloy with application potential due to its tuneable antibacterial action (silver) and its optical properties for bioimaging.

 Please wait while we load your content...
Please wait while we load your content...