Biodegradable glycopolymer-b-poly(ε-caprolactone) block copolymer micelles: versatile construction, tailored lactose functionality, and hepatoma-targeted drug delivery†
Abstract
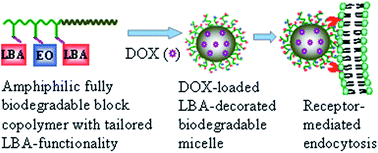
Glycopolymer-b-poly(ε-caprolactone) (GP-PCL) block copolymer micelles (‘glycomicelles’) with tailored lactose functionalities were developed and investigated for hepatoma-targeted doxorubicin (DOX) delivery. Amphiphilic GP-PCL copolymers were readily prepared with controlled lactobionic acid (LBA) functionalities of 20%, 40%, 80%, and 100% (denoted as GP20-PCL, GP40-PCL, GP80-PCL, and GP100-PCL, respectively) through post-polymerization modification of the poly(acryloyl cyclic carbonate)-b-poly(ε-caprolactone) (PAC-b-PCL, 11.6–6.4 kg mol−1) block copolymer with thiolated LBA (LBA-SH) and 2-(2-methoxyethoxy)ethanethiol ((EO)2-SH) via the Michael-type addition reaction. These self-assembled glycomicelles had mean hydrodynamic diameters ranging from 31.9 to 76.8 nm depending on LBA densities, and exhibited high DOX loading efficiencies of 83.0–89.2%. In vitro release studies showed that the DOX release rate depended on the pH and LBA content. Flow cytometric analyses revealed that asialoglycoprotein receptor (ASGP-R) over-expressed HepG2 liver cancer cells following 4 h treatment with DOX-loaded glycomicelles had a 6.6–17.1-fold higher DOX level, depending on LBA densities, as compared to those treated with the corresponding DOX-loaded non-glycomicelles (100% substitution with (EO)2-SH) under otherwise the same conditions. MTT assays demonstrated that DOX-loaded GP20-PCL, GP40-PCL, GP80-PCL and GP100-PCL micelles had much lower half maximal inhibitory concentration (IC50) values of 2.05, 0.75, 0.45 and 0.43 μg DOX equiv. mL−1, respectively, in HepG2 cells than DOX-loaded non-glycomicelles (IC50: 6.55 μg mL−1 DOX equiv. mL−1). Competitive inhibition experiments showed that after the incubation with DOX-loaded glycomicelles for 4 h, more efficient killing activity against free HepG2 cells (−LBA) was observed, as compared to that against LBA-blocked HepG2 cells (+LBA) after a subsequent 72 h incubation. Glycomicelles with tailored LBA functionalities, high drug loading capacity, and high uptake by ASGP-R positive cells are promising candidates for liver cancer chemotherapy.
- This article is part of the themed collection: JMC B Top Picks collection: Recent advances in drug delivery

 Please wait while we load your content...
Please wait while we load your content...