Effect of nickel oxidation state on the structural and electrochemical characteristics of lithium-rich layered oxide cathodes†
Abstract
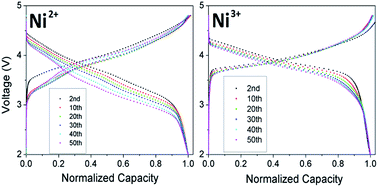
Li-rich layered oxides are being pursued intensively as next generation cathodes for Li-ion batteries due to their high capacity, but their adoption is hampered by voltage decay during cycling. This investigation focuses on the effect of Ni oxidation state on the structural and electrochemical properties, including voltage decay, of Li-rich layered oxides. With increasing Ni3+ content, (i) the interlayer mixing between Li and Ni (cation disorder) decreases due to its smaller size compared to Ni2+, (ii) the oxygen loss plateau decreases in length due the decreased Mn4+ content and Li content in the transition-metal layer, resulting in a reduction in Li2MnO3 character, (iii) the discharge capacity decreases due to the shorter plateau and a larger 1st cycle irreversible capacity loss, (iv) the operating voltage increases due to the increasing amount of Ni and as Ni4+/3+ reduction occurs at a higher voltage than Mn4+ reduction, and (v) cyclability is enhanced as less Mn3+ ions are formed, minimizing Mn dissolution. Most importantly, the decreasing degree of Mn reduction suppresses the layered-to-spinel phase transformation and the consequent voltage decay. Increasing the oxidation state of Ni could thus be a potential approach to reduce the voltage decay in lithium-rich layered oxides, but it would cause a decrease in capacity.

 Please wait while we load your content...
Please wait while we load your content...