Nickel-rich layered LiNi1−xMxO2 (M = Mn, Fe, and Co) electrocatalysts with high oxygen evolution reaction activity†
Abstract
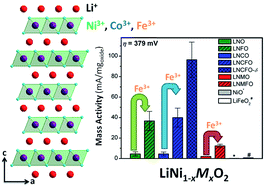
An understanding of the materials characteristics that lead to high electrocatalytic activity for the oxygen evolution reaction (OER) is needed to make electrolytic hydrogen fuel production and rechargeable metal-air batteries a reality. Here, the first systematic investigation of a family of Ni-rich layered LiNi1−xMxO2 (M = Mn, Fe, and Co) oxides reveals that the catalytic activity can be tuned by varying the Ni content, nature of the transition-metal dopant, lithium content, and degree of cation ordering between Li and Ni/M. In particular, Fe-doping in LiNi1−xMxO2 imparts the most dramatic improvements in OER activity, possibly due to the flexibility of Fe to adopt different coordination geometries on the surface. X-ray photoelectron spectroscopic (XPS) data reveal that the surface of the Fe-doped sample is enriched with Fe while ex situ Raman spectroscopy indicates that the layered morphology is preserved during electrochemical cycling, but the cation disorder increases. Among the various LiNi1−xMxO2 compositions investigated, LiNi0.7Co0.3Fe0.2O2 exhibits the highest OER activity, which increases further when excess lithium and oxygen vacancies are present, and good stability. The Ni-rich LiNi1−xMxO2 samples join a growing number of highly active iron-doped systems for OER electrocatalysis in alkaline conditions.

 Please wait while we load your content...
Please wait while we load your content...