Fabrication of nitrogen-doped graphite felts as positive electrodes using polypyrrole as a coating agent in vanadium redox flow batteries†
Abstract
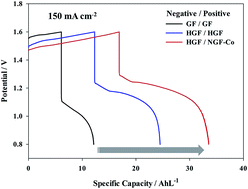
A novel method for preparing nitrogen-doped graphite felts as positive electrodes in vanadium redox flow batteries was developed and studied. These materials were synthesized by directly coating a thin polypyrrole layer on the graphite felt surface followed by subsequent carbonization in the presence of Co (NGF-Co). Co played an important role in promoting the concentration of nitrogen functional groups that are active towards the VO2+/VO2+ redox couple. The physical and electrochemical properties of the nitrogen-doped carbons were characterized by X-ray photoelectron spectrometry, cyclic voltammetry, impedance spectroscopy and charge–discharge tests. These results indicate that synthesizing NGF-Co via this facile method substantially improved the electrochemical catalytic activity and reduced charge transfer resistance. It exhibited a 211% and 34% increase in discharge capacity over the pristine graphite felt and thermally oxidized graphite felt, respectively, at a high current density of 150 mA cm−2. The enhanced performance was attributed to the abundant active nitrogen groups created on the graphite felt, which benefit the fast electrochemical kinetics of vanadium redox reactions.

 Please wait while we load your content...
Please wait while we load your content...