Tailoring molecular architectures of Fe phthalocyanine on nanocarbon supports for high oxygen reduction performance†
Abstract
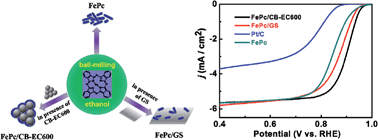
Cost-effective non-precious metal electrocatalysts for the oxygen reduction reaction (ORR) is the key for fuel cells to become a viable electricity generation technology. Metal macrocyclic compounds such as Fe/Co porphyrins and phthalocyanines are excellent molecular catalysts for O2 reduction; but they are still considerably less competitive than Pt-based materials when catalyzing the ORR in electrochemical environments. Using Fe phthalocyanine (FePc) as a model compound, we show that the electrocatalytic activity of metal macrocyclic compounds for the ORR can be greatly enhanced through tailoring assembling architectures on high-surface-area nanocarbons. By simply ball-milling FePc with nanocarbons, such as graphene nanosheets and carbon-black nanoparticles, molecular architectures of FePc from nanorods to uniform thin shells are obtained. The resulting carbon-supported FePc composites exhibit ORR performance much superior to the state-of-the-art carbon-supported Pt in alkaline solution, with up to a 60 mV positive shift in the half-wave potential and more than 5 times increase in the mass activity. As well as showing that the molecule–support interaction provides a degree of control on the molecular architectures of metal macrocyclic compounds, the present work reveals that the FePc molecule is intrinsically much more efficient than Pt in catalyzing the ORR in alkaline media, and therefore has great prospects as a cathode electrocatalyst in alkaline fuel cells.

 Please wait while we load your content...
Please wait while we load your content...