Enhanced reactive adsorption of H2S on Cu–BTC/ S- and N-doped GO composites†
Abstract
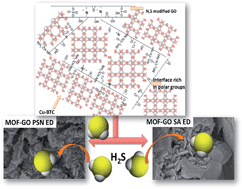
New composites containing Cu–BTC and S- and N-doped graphite oxides (GOs) were synthesized. The composites were evaluated as adsorbents of H2S under ambient conditions. The texture and chemistry of the initial samples and those exposed to H2S were analyzed using a wide range of analytical techniques (XRD, SEM-EDX, FTIR, thermal analysis-MS, and nitrogen adsorption). The performance of the new composites as H2S adsorbents was much better than that of the parent MOFs. It was owing to the formation of new microporosity as a result of linkages between the sulfonic acids and amine groups of modified GO and copper centers Cu–BTC. The presence of moisture in the pore system increased the amount adsorbed. Physical adsorption and reactive adsorption play an important role in the mechanism of retention. The removal of H2S is favored on the composites with a higher degree of surface heterogeneity, which facilitates the retention of H2S molecules mostly in the form of sulfides. Given the differences in the chemistry of the N- and S-containing groups in the composites, distinct mechanisms of adsorption occur, which result in sulfides/sulfates of unique morphologies. Nitrogen functional groups catalyze the formation of superoxide ions on the graphene phase, resulting in the partial oxidation of H2S and in the release of SO2.

 Please wait while we load your content...
Please wait while we load your content...