Acid Orange II degradation through a heterogeneous Fenton-like reaction using Fe–TiO2 nanotube arrays as a photocatalyst
Abstract
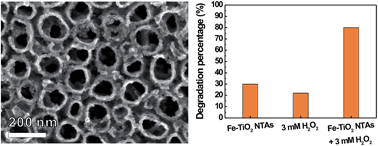
The photocatalytic degradation of the non-biodegradable azo dye Acid Orange II (AO II) was performed via a heterogeneous Fenton-like process using Fe incorporated TiO2 nanotube arrays (Fe–TiO2 NTAs) as a photocatalyst in the presence of H2O2 under UV irradiation. Fe–TiO2 NTAs were prepared by an ultrasound-assisted impregnating-calcination method. The effects of reaction conditions including the amount of Fe incorporated into TiO2 NTAs, initial concentration of H2O2, and initial pH of the AO II solution on the photocatalytic degradation rate of AO II were investigated. The results revealed that the Fe–TiO2 NTAs prepared by ultrasonic deposition for 25 min exhibited the highest photocatalytic activity compared with pure TiO2 NTA and other Fe–TiO2 NTA samples. In the presence of H2O2, the photocatalytic degradation rate of AO II was significantly enhanced. About 80% AO II can be decomposed at pH 2.96 by using Fe–TiO2 NTAs prepared by ultrasonic deposition for 25 min as a photocatalyst with 3 mM H2O2 after 120 min of UV irradiation, which was 1.7 times higher than that without H2O2 (∼30%). Very interestingly, there was an obvious synergistic effect between the photocatalytic process of Fe–TiO2 NTAs and H2O2 oxidation. Furthermore, the Fe–TiO2 NTA photocatalyst exhibited excellent stability in the heterogeneous Fenton-like process. Thus, we demonstrated that the heterogeneous Fenton-like process combining the Fe–TiO2 NTA photocatalyst with H2O2 oxidation has a promising application in practical wastewater treatment.

 Please wait while we load your content...
Please wait while we load your content...