Enhanced adsorption removal of methyl orange from aqueous solution by nanostructured proton-containing δ-MnO2†
Abstract
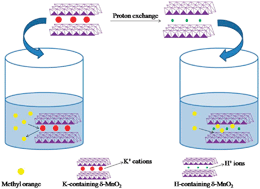
Two nanostructured proton-containing δ-MnO2 (H-δ-MnO2) materials were synthesized through proton exchange for K-containing δ-MnO2 (K-δ-MnO2) nanosheets and nanoparticles prepared by the hydrothermal homogeneous precipitation method and solid-state reaction. Energy-dispersive spectroscopy (EDS) measurements showed that the K+ cations were successfully leached away and replaced with H+ ions. The role of K+ cations in the adsorption process was first explored by replacing the K+ ions in the δ-MnO2 with H+ ions and the adsorption capacities for organic pollutants were investigated using methyl orange (MO) as a model. Experimental results indicated that the H-δ-MnO2 materials with a similar morphology and crystal structure showed excellent adsorption capacities of 375 and 427 mg g−1 for MO, because both of the nanostructured H-δ-MnO2 materials have much larger Brunauer–Emmett–Teller (BET) surface areas than that of K-δ-MnO2. The remarkable adsorption capacities of dye onto H-δ-MnO2 materials indicate that K+ cations in the layered δ-MnO2 structure have an unfavorable influence on the adsorption process. Moreover, physical adsorption mechanisms including electrostatic interaction play a dominant role in the adsorption mechanism between MO and adsorbents. This finding suggests that the proton-exchange process may be a useful method to improve the adsorption affinity of dye contaminants on δ-MnO2 and nanostructured proton-containing δ-MnO2 samples are good candidates for efficient MO removal from wastewater.

 Please wait while we load your content...
Please wait while we load your content...