Photofuel cell comprising titanium oxide and bismuth oxychloride (BiO1−xCl1−y) photocatalysts that uses acidic water as a fuel†
Abstract
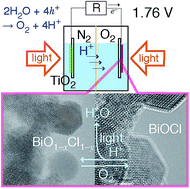
Solar cells and fuel cells have been extensively investigated. However, the need for sustainability, durability, and an electromotive force greater than 1.5 V per cell has not yet been fully realized. Herein, a photofuel cell (PFC) comprising two photocatalysts TiO2 and BiOCl that uses acidic water as a fuel is described. The PFC is designed such that water can be regenerated from the photogenerated O2 in the cell, and the electromotive force is as high as the difference between the conduction band minimum of TiO2 and the valence band maximum of BiOCl (theoretical: 2.75 V, experimental: 1.76 V). The photocurrents were in accordance with a kinetic model based on the combination of the excited electron concentration at TiO2 and the hole concentration at BiOCl. The reaction between the surface sites of O and/or Cl-deficient BiO1−xCl1−y species formed by ultraviolet-visible light irradiation with O2 was in situ monitored using Bi L3-edge extended X-ray absorption fine structure and X-ray photoelectron spectroscopy supported by Raman and UV-visible spectroscopy and could be involved in the cathodic O2 photoreduction in the PFC. Furthermore, amorphous-like BiO1−xCl species were observed on the surface of the BiOCl crystallites in high-resolution transmission electron microscopy. Importantly, this PFC that comprises TiO2 and BiOCl is sustainable, recyclable, and has a series resistance that can be controlled by adjusting the BiOCl particle size, and exhibits a more efficient charge flow because of band bending than that of a previously reported PFC based on the rectification of the Schottky barrier.


 Please wait while we load your content...
Please wait while we load your content...