MIL-101(Fe) as a lithium-ion battery electrode material: a relaxation and intercalation mechanism during lithium insertion†
Abstract
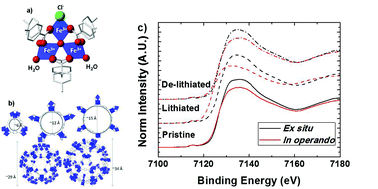
The electrochemical performance of a MIL-101(Fe) metal–organic framework (MOF) as a lithium ion battery electrode is reported for the first time. Iron metal centers can be electrochemically activated. The Fe3+/Fe2+ redox couple is electrochemically active, but not reversible over many cycles. A comparison between ex situ and in operando X-ray absorption spectroscopy (XAS) on the Fe K-edge is presented. Our results indicate that the capacity fade is related to a time dependent, irreversible oxidation of Fe2+ to Fe3+. These results are key in proving the importance of in operando XAS measurements. The MOF side reaction with an electrolyte has been computationally modeled. These results provide further insights on the mechanism responsible for the MOF lack of reversibility. Future guidelines for improving the reversibility of MOFs used as electrodes in Li-ion batteries based on the fine-tuning of the electronic structure of the material are proposed.

 Please wait while we load your content...
Please wait while we load your content...