Highly branched cobalt phosphide nanostructures for hydrogen-evolution electrocatalysis†
Abstract
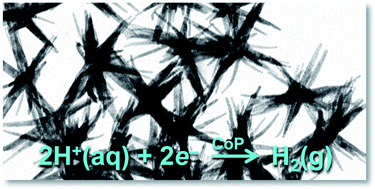
CoP nanostructures that exposed predominantly (111) crystal facets were synthesized and evaluated for performance as electrocatalysts for the hydrogen-evolution reaction (HER). The branched CoP nanostructures were synthesized by reacting cobalt(II) acetylacetonate with trioctylphosphine in the presence of trioctylphosphine oxide. Electrodes comprised of the branched CoP nanostructures deposited at a loading density of ∼1 mg cm−2 on Ti electrodes required an overpotential of −117 mV to produce a current density of −20 mA cm−2 in 0.50 M H2SO4. Hence the branched CoP nanostructures belong to the growing family of highly active non-noble-metal HER electrocatalysts. Comparisons with related CoP systems have provided insights into the impact that shape-controlled nanoparticles and nanoparticle–electrode interactions have on the activity and stability of nanostructured HER electrocatalysts.

 Please wait while we load your content...
Please wait while we load your content...