Fabrication of hollow Cu2O@CuO-supported Au–Pd alloy nanoparticles with high catalytic activity through the galvanic replacement reaction†
Abstract
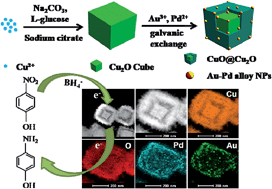
We report the synthesis of unique Au–Pd bimetallic alloy nanoparticles (NPs) supported on a hollow Cu2O–CuO core–shell heterostructure via the galvanic replacement method, which represents the first example of Au–Pd alloy nanoparticles immobilized on hollow Cu2O@CuO nanocubes. The morphological evolution can be controlled by the reaction time. The morphology, composition, and structure of the as-prepared Cu2O@CuO/(Au–Pd) material are characterized by scanning electron microscopy (SEM), transmission electron microscopy (TEM), high resolution transmission electron microscopy (HRTEM), X-ray powder diffraction (XRD), X-ray photoelectron spectroscopy (XPS), elemental analysis (EDS) and inductively coupled plasma atomic emission spectroscopy (ICP-AES). The size of the Au–Pd bimetallic NPs deposited onto the Cu2O@CuO nanocubes is ca. 15 nm. These bimetallic NPs showed a high catalytic activity towards the reduction of 4-nitrophenol (4-NP) to 4-aminophenol (4-AP) in water at room temperature. Significantly, the strong synergistic effect between Au and Pd, and the high specific surface area of Cu2O@CuO enhance the catalytic efficiency. The catalytic system can be reused for several cycles without any obvious decay of the catalytic efficiency.

 Please wait while we load your content...
Please wait while we load your content...