Using intermolecular interactions to crosslink PIM-1 and modify its gas sorption properties†
Abstract
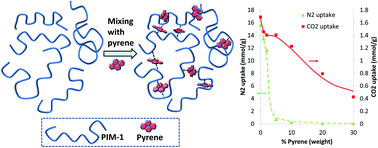
The intermolecular interactions between the “polymer of intrinsic microporosity” PIM-1 and polycyclic aromatic hydrocarbons (PAHs) have been investigated with the aim of modifying the gas sorption and physical properties. Mixing PIM-1 with selected PAHs resulted in rapid precipitation of polymer. Blending PIM-1 with pyrene had a significant effect of the gas sorption properties of the resulting films; dramatically reduced N2 uptake (77 K), whilst CO2 uptake at 298 K was only slightly reduced. A gate-opening behaviour was also observed for the N2 gas sorption (77 K), which was related to the pyrene content of the blend. Using an electron-donating PAH as the additive resulted in a stronger interaction. By exploiting a post-modification strategy after PIM-1 film formation, the absorption of either pyrene or 1-aminopyrene produced films with higher elastic moduli and greatly improved CO2/N2 gas sorption selectivities (293 K). Single gas permeability measurements revealed that while the 1-aminopyrene modified film possessed reduced CO2 permeability, it possessed enhanced CO2/N2 selectivity. Importantly, the ageing of the permeability was halted over the 50 days tested, likely due to the physical crosslinking of the polymer chains by 1-aminopyrene.
- This article is part of the themed collection: Highlighting materials research in the UK for energy and sustainability

 Please wait while we load your content...
Please wait while we load your content...