Facile fabrication of cost-effective porous polymer networks for highly selective CO2 capture†
Abstract
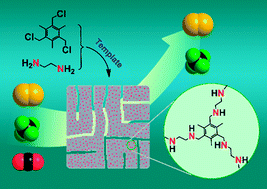
Due to their synthetic diversification, low skeletal density, and high physicochemical stability, porous polymer networks (PPNs) are highly promising in a variety of applications such as carbon capture. Nevertheless, complicated monomers and/or expensive catalysts are normally utilized for their synthesis, which makes the process tedious, costly, and hard to scale up. In this study, a facile nucleophilic substitution reaction was designed to fabricate PPNs from low-cost monomers, namely chloromethyl benzene and ethylene diamine. A surfactant template was also used to direct the assembly, leading to the formation of PPN with enhanced porosity. It is fascinating that the polymerization reactions can occur at the low temperature of 63 °C in the absence of any catalyst. The obtained PPNs contain abundant secondary amines, which offer appropriate adsorbate–adsorbent interactions from the viewpoints of selective CO2 capture and energy-efficient regeneration of the adsorbents. Hence, these PPNs are highly active in selective adsorption of CO2, and unusually high CO2/N2 and CO2/CH4 selectivity was obtained. Moreover, the PPN adsorbents can be completely regenerated under mild conditions.

 Please wait while we load your content...
Please wait while we load your content...