Ni-doped Mo2C nanowires supported on Ni foam as a binder-free electrode for enhancing the hydrogen evolution performance†
Abstract
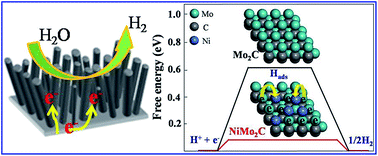
In this study, an inexpensive electrocatalyst, Ni-doped Mo2C nanowires, were grown directly on Ni foam via a hydrothermal reaction combined with a carburization process. X-ray diffraction (XRD), field-emission scanning electron microscopy (FE-SEM), transmission electron microscopy (TEM), X-ray photoelectron spectroscopy (XPS), cyclic voltammetry (CV), and linear scanning voltammetry (LSV) were used to scrutinize the catalysts and their electrochemical performance. The results showed that the designed NiMo2C/NF catalyst displays enhanced catalytic activity toward hydrogen production with a low onset overpotential of 21 mV. For driving a cathodic current density of 100 mA cm−2, it only needs an overpotential of 150 mV. Such excellent performance of NiMo2C/NF could be ascribed to the high intrinsic activity from a synergistic function of Ni and Mo2C, as well as to the exposure of more Ni-doped Mo2C sites provided by the high aspect ratio of a one-dimensional (1D) structure and rich surface area.

 Please wait while we load your content...
Please wait while we load your content...