Synthesis of high-silica AEI zeolites with enhanced thermal stability by hydrothermal conversion of FAU zeolites, and their activity in the selective catalytic reduction of NOx with NH3†
Abstract
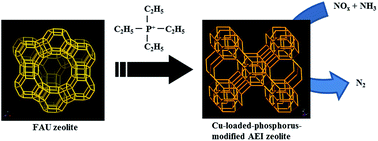
High-silica AEI zeolites with Si/Al ratios of 13–20 were synthesized by FAU interzeolite conversion, in which tetraethylphosphonium (TEP) cations as a structure-directing agent (SDA) and NaF or NH4F as a fluoride source were added to the starting gels. During the thermal treatment of the AEI zeolites, TEP cations in the zeolitic pores decomposed to yield the P-modified AEI zeolites with various P/Al ratios. The thermal stability of the P-modified AEI zeolite was higher than that of the non-modified AEI zeolite synthesized using the N,N-diethyl-2,6-dimethylpiperidinium cation as an SDA. The framework structure of the P-modified AEI zeolite was maintained after calcination at 1000 °C for 1 h, indicating an enhanced thermal stability by phosphorus modification. The catalytic performance of Cu-loaded AEI zeolites with different P/Al ratios in the selective catalytic reduction (SCR) of NOx with NH3 was also investigated. The NO conversion was found to increase with decreasing P/Al ratio. No decrease in the NO conversion was observed after hydrothermal treatment at 900 °C for 4 h, indicating the high potential of the P-modified AEI zeolite for NH3-SCR of NOx.

 Please wait while we load your content...
Please wait while we load your content...