Vertically aligned nanocomposite La0.8Sr0.2CoO3/(La0.5Sr0.5)2CoO4 cathodes – electronic structure, surface chemistry and oxygen reduction kinetics†
Abstract
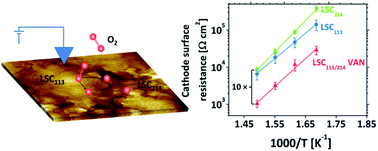
The hetero-interfaces between the perovskite (La1−xSrx)CoO3 (LSC113) and the Ruddlesden-Popper (La1−xSrx)2CoO4 (LSC214) phases have recently been reported to exhibit fast oxygen exchange kinetics. Vertically aligned nanocomposite (VAN) structures offer the potential for embedding a high density of such special interfaces in the cathode of a solid oxide fuel cell in a controllable and optimized manner. In this work, VAN thin films with hetero-epitaxial interfaces between LSC113 and LSC214 were prepared by pulsed laser deposition. In situ scanning tunneling spectroscopy established that the LSC214 domains in the VAN structure became electronically activated, by charge transfer across interfaces with adjacent LSC113 domains above 250 °C in 10−3 mbar of oxygen gas. Atomic force microscopy and X-ray photoelectron spectroscopy analysis revealed that interfacing LSC214 with LSC113 also provides for a more stable cation chemistry at the surface of LSC214 within the VAN structure, as compared to single phase LSC214 films. Oxygen reduction kinetics on the VAN cathode was found to exhibit approximately a 10-fold enhancement compared to either single phase LSC113 and LSC214 in the temperature range of 320–400 °C. The higher reactivity of the VAN surface to the oxygen reduction reaction is attributed to enhanced electron availability for charge transfer and the suppression of detrimental cation segregation. The instability of the LSC113/214 hetero-structure surface chemistry at temperatures above 400 °C, however, was found to lead to degraded ORR kinetics. Thus, while VAN structures hold great promise for offering highly ORR reactive electrodes, efforts towards the identification of more stable hetero-structure compositions for high temperature functionality are warranted.

 Please wait while we load your content...
Please wait while we load your content...