Ion exchange in alginate gels – dynamic behaviour revealed by electron paramagnetic resonance†
Abstract
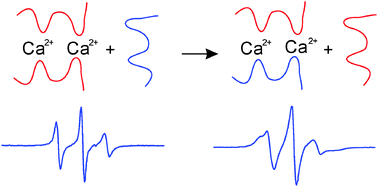
The formation of alginate gel from low molecular weight alginate and very low molecular weight alginate in the presence of divalent cations was investigated using Electron Paramagnetic Resonance (EPR) spectroscopy. The transition from sol to gel in the presence of divalent cations was monitored by the changes in the dynamics of spin labelled alginate. The immobilisation of the spin labelled alginate in the gel reflects the strength of interaction between the cation and alginate chain. Diffusion experiments showed that both the cation and alginate polyanion in the gel fibres can exchange with molecules in solution. In particular, we showed that dissolved alginate polyanions can replace alginates in the gel fibres, which can hence diffuse through the bulk of the gel. This illustrates the surprisingly highly dynamic nature of these gels and opens up the possibility of preparing multicomponent alginate gels via polyanion exchange process.

 Please wait while we load your content...
Please wait while we load your content...