Tuning the mesomorphic properties of phenoxy-terminated smectic liquid crystals: the effect of fluoro substitution†
Abstract
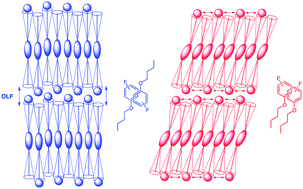
The mesomorphic properties of phenoxy-terminated 5-alkoxy-2-(4-alkoxyphenyl)pyrimidine liquid crystals can be tuned in a predictable fashion with fluoro substituents on the phenoxy end-group. We show that an ortho-fluoro substituent promotes the formation of a tilted smectic C (SmC) phase whereas a para-fluoro substituent promotes the formation of an orthogonal smectic A (SmA) phase. The balance between SmA and SmC phases may be understood in terms of the energetic preference of the phenoxy end-groups to self-assemble via arene–arene interactions in a parallel or antiparallel geometry, and how these non-covalent interactions may cause either a suppression or enhancement of out-of-layer fluctuations at the interface of smectic layers. Calculations of changes in the potential energy of association ΔE for non-covalent dimers of fluoro-substituted n-butyloxybenzene molecules in parallel and antiparallel geometries support this hypothesis. We also show how mesomorphic properties can be further tuned by difluoro and perfluoro substitution, including difluoro substitution at the ortho positions, which uniquely promotes the formation of a SmC-nematic phase sequence.

 Please wait while we load your content...
Please wait while we load your content...