Fusion of raft-like lipid bilayers operated by a membranotropic domain of the HSV-type I glycoprotein gH occurs through a cholesterol-dependent mechanism†
Abstract
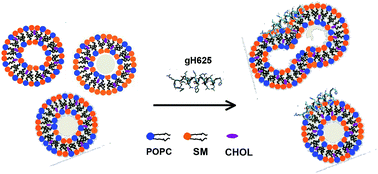
A wealth of evidence indicates that lipid rafts are involved in the fusion of the viral lipid envelope with the target cell membrane. However, the interplay between these sterol- and sphingolipid-enriched ordered domains and viral fusion glycoproteins has not yet been clarified. In this work we investigate the molecular mechanism by which a membranotropic fragment of the glycoprotein gH of the Herpes Simplex Virus (HSV) type I (gH625) drives fusion of lipid bilayers formed by palmitoyl oleoyl phosphatidylcholine (POPC)–sphingomyelin (SM)–cholesterol (CHOL) (1 : 1 : 1 wt/wt/wt), focusing on the role played by each component. The comparative analysis of the liposome fusion assays, Dynamic Light Scattering (DLS), spectrofluorimetry, Neutron Reflectivity (NR) and Electron Spin Resonance (ESR) experiments, and Molecular Dynamics (MD) simulations shows that CHOL is fundamental for liposome fusion to occur. In detail, CHOL stabilizes the gH625-bilayer association by specific interactions with the peptide Trp residue. The interaction with gH625 causes an increased order of the lipid acyl chains, whose local rotational motion is significantly hampered. SM plays only a minor role in the process, favoring the propagation of lipid perturbation to the bilayer inner core. The stiffening of the peptide-interacting bilayer leaflet results in an asymmetric perturbation of the membrane, which is locally destabilized thus favoring fusion events. Our results show that viral fusion glycoproteins are optimally suited to exert a high fusogenic activity on lipid rafts and support the relevance of cholesterol as a key player of membrane-related processes.

 Please wait while we load your content...
Please wait while we load your content...