Galvanic replacement synthesis of AgxAu1−x@CeO2 (0 ≤ x ≤ 1) core@shell nanospheres with greatly enhanced catalytic performance†
Abstract
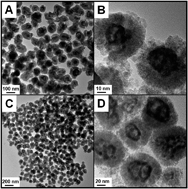
A galvanic replacement strategy has been successfully adopted to design AgxAu1−x@CeO2 core@shell nanospheres derived from Ag@CeO2 ones. After etching using HAuCl4, the Ag core was in situ replaced with AgxAu1−x alloy nanoframes, and void spaces were left under the CeO2 shell. Among the as-prepared AgxAu1−x@CeO2 catalysts, Ag0.64Au0.36@CeO2 shows the optimal catalytic performance, whose catalytic efficiency reaches even 2.5 times higher than our previously reported Pt@CeO2 nanospheres in the catalytic reduction of 4-nitrophenol (4-NP) by ammonia borane (AB). Besides, Ag0.64Au0.36@CeO2 also exhibits a much lower 100% conversion temperature of 120 °C for catalytic CO oxidation compared with the other samples.
- This article is part of the themed collection: Global Energy Challenges: Hydrogen Energy

 Please wait while we load your content...
Please wait while we load your content...