Ring-chain synergy in ionic liquid electrolytes for lithium batteries†
Abstract
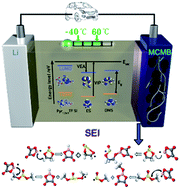
Lithium-ion batteries have been attracting much attention which enables the revolution of wireless global communication. Ionic liquids are regarded as promising candidates for lithium-ion battery electrolytes because they can overcome the limitations of high operating temperatures and flammability concerns of traditional electrolytes. However, at low temperatures they suffer from low ionic conductivity and phase transition. In this paper mixed electrolyte systems are described based on N-methoxyethyl-N-methylpyrrolidinium bis(trifluoromethanesulfonyl)-imide (Pyr1,2O1TFSI) and lithium difluoro(oxalate)borate (LiODFB) lithium salt, with ethylene sulphite (ES) or dimethyl sulphite (DMS) as a cosolvent. The mixed electrolyte system exhibits good ion transport properties (a conductivity of 8.163 mS cm−1), a wide electrochemical window (5.2 V), non-flammability, the ability to form films to protect the anode and a large operating temperature range (−40 °C to 60 °C). We compare the performance and function of the new mixed electrolyte system with a variety of ionic liquid/cosolvent electrolyte systems developed in previous studies. The ring-chain synergy takes advantage of the availability of both high permittivities based on the ring-like components and low viscosities based on the chain-like components in the mixed electrolyte system and causes the electrolyte to exhibit a good overall performance in safety, ion transport and compatibility with electrodes.
- This article is part of the themed collections: Global Energy Challenges: Electrochemical Energy and Global Energy Challenges: Energy Applications


 Please wait while we load your content...
Please wait while we load your content...