Anion-templated hexagonal nanotubes†
Abstract
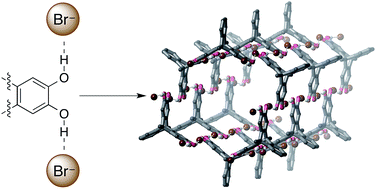
Hydrogen bonding between bromide anions and a tetrahydroxytriptycene ligand was used to assemble crystalline hexagonal tubes with nanometer diameters in good yield. Use of a hexahydroxytriptycene ligand again gave hexagonal nanotubes, but containing the spontaneously-oxidised quinone–tetrahydroxy ligand. The surprisingly robust nanotubes are stable to heat, vacuum and water, and represent an unprecedented use of O–H⋯anion coordination to assemble complex three-dimensional structures.


 Please wait while we load your content...
Please wait while we load your content...