Non-classical selectivities in the reduction of alkenes by cobalt-mediated hydrogen atom transfer†
Abstract
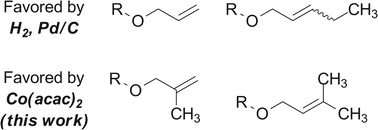
Classical methods for alkene hydrogenation typically reduce less-substituted or more-strained alkenes, or those in proximity to a directing group, most rapidly. Here we describe a cobalt-mediated hydrogenation protocol that provides complementary selectivities in the reduction of several classes of olefins and alkynes. The selectivity of this reduction derives from a hydrogen atom transfer mechanism, which favors the generation of the more stable alkylradical intermediate. We also report the first alkene hydrobromination, hydroiodination, and hydroselenylation by a hydrogen atom transfer process.

 Please wait while we load your content...
Please wait while we load your content...