Well-defined silica supported aluminum hydride: another step towards the utopian single site dream?†
Abstract
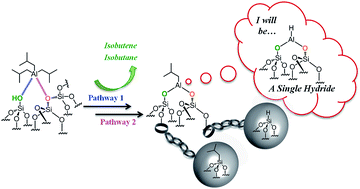
Reaction of triisobutylaluminum with SBA15700 at room temperature occurs by two parallel pathways involving either silanol or siloxane bridges. It leads to the formation of a well-defined bipodal [(![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) SiO)2Al–CH2CH(CH3)2] 1a, silicon isobutyl [
SiO)2Al–CH2CH(CH3)2] 1a, silicon isobutyl [![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si–CH2CH(CH3)2] 1b and a silicon hydride [
Si–CH2CH(CH3)2] 1b and a silicon hydride [![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si–H] 1c. Their structural identity was characterized by FT-IR and advanced solid-state NMR spectroscopies (1H, 13C, 29Si, 27Al and 2D multiple quantum), elemental and gas phase analysis, and DFT calculations. The reaction involves the formation of a highly reactive monopodal intermediate: [
Si–H] 1c. Their structural identity was characterized by FT-IR and advanced solid-state NMR spectroscopies (1H, 13C, 29Si, 27Al and 2D multiple quantum), elemental and gas phase analysis, and DFT calculations. The reaction involves the formation of a highly reactive monopodal intermediate: [![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) SiO–Al–[CH2CH(CH3)2]2], with evolution of isobutane. This intermediate undergoes two parallel routes: transfer of either one isobutyl fragment or of one hydride to an adjacent silicon atom. Both processes occur by opening of a strained siloxane bridge,
SiO–Al–[CH2CH(CH3)2]2], with evolution of isobutane. This intermediate undergoes two parallel routes: transfer of either one isobutyl fragment or of one hydride to an adjacent silicon atom. Both processes occur by opening of a strained siloxane bridge, ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) Si–O–Si
Si–O–Si![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) but with two different mechanisms, showing that the reality of “single site” catalyst may be an utopia: DFT calculations indicate that isobutyl transfer occurs via a simple metathesis between the Al-isobutyl and O–Si bonds, while hydride transfer occurs via a two steps mechanism, the first one is a β-H elimination to Al with elimination of isobutene, whereas the second is a metathesis step between the formed Al–H bond and a O–Si bond. Thermal treatment of 1a (at 250 °C) under high vacuum (10−5 mbar) generates Al–H through a β-H elimination of isobutyl fragment. These supported well-defined Al–H which are highly stable with time, are tetra, penta and octa coordinated as demonstrated by IR and 27Al–1H J-HMQC NMR spectroscopy. All these observations indicate that surfaces atoms around the site of grafting play a considerable role in the reactivity of a single site system.
but with two different mechanisms, showing that the reality of “single site” catalyst may be an utopia: DFT calculations indicate that isobutyl transfer occurs via a simple metathesis between the Al-isobutyl and O–Si bonds, while hydride transfer occurs via a two steps mechanism, the first one is a β-H elimination to Al with elimination of isobutene, whereas the second is a metathesis step between the formed Al–H bond and a O–Si bond. Thermal treatment of 1a (at 250 °C) under high vacuum (10−5 mbar) generates Al–H through a β-H elimination of isobutyl fragment. These supported well-defined Al–H which are highly stable with time, are tetra, penta and octa coordinated as demonstrated by IR and 27Al–1H J-HMQC NMR spectroscopy. All these observations indicate that surfaces atoms around the site of grafting play a considerable role in the reactivity of a single site system.

 Please wait while we load your content...
Please wait while we load your content...