Importance of dipole moments and ambient polarity for the conformation of Xaa–Pro moieties – a combined experimental and theoretical study†
Abstract
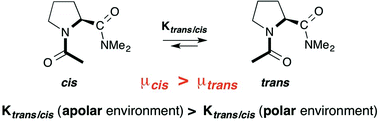
NMR spectroscopic studies with a series of proline derivatives revealed that the polarity of the environment has a significant effect on the trans : cis isomer ratio of Xaa–Pro bonds. Computational studies showed that this effect is due to differences in the overall dipole moments of trans and cis conformers. Comparisons between the conformational properties of amide and ester derivatives revealed an intricate balance between polarity effects and n → π* interactions of adjacent carbonyl groups. The findings have important implications for protein folding and signaling as well as the performance of proline-based stereoselective catalysts.
- This article is part of the themed collections: Celebrating the 2016 RSC Prize and Award Winners and ISACS16: Challenges in Chemical Biology

 Please wait while we load your content...
Please wait while we load your content...