Unstrained C–C bond activation and directed fluorination through photocatalytically-generated radical cations†
Abstract
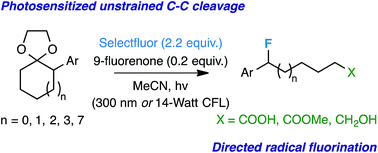
Expanding the repertoire of controlled radical fluorination techniques, we present a photosensitized unstrained C–C bond activation/directed monofluorination method using Selectfluor and 9-fluorenone. The reaction is amenable to the opening of multiple 1-acetal-2-aryl substituted rings to yield ω-fluoro carboxylic acids, esters, alcohols, and ketones with relative ease. Initial mechanistic insight suggests radical ion intermediates.

 Please wait while we load your content...
Please wait while we load your content...