Highly enantioselective construction of tertiary thioethers and alcohols via phosphine-catalyzed asymmetric γ-addition reactions of 5H-thiazol-4-ones and 5H-oxazol-4-ones: scope and mechanistic understandings†
Abstract
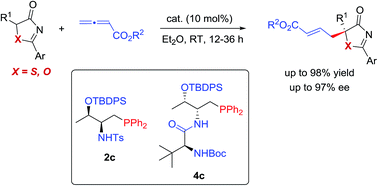
Phosphine-catalyzed highly enantioselective γ-additions of 5H-thiazol-4-ones and 5H-oxazol-4-ones to allenoates have been developed for the first time. With the employment of amino-acid derived bifunctional phosphines, a wide range of substituted 5H-thiazol-4-one and 5H-oxazol-4-one derivatives bearing heteroatom (S or O)-containing tertiary chiral centers were constructed in high yields and excellent enantioselectivities. The reported method provides facile access to enantioenriched tertiary thioethers/alcohols. The mechanism of the γ-addition reaction was investigated by performing DFT calculations, and the hydrogen bonding interactions between the Brønsted acid moiety of the phosphine catalysts and the “C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O” unit of the donor molecules were shown to be crucial in asymmetric induction.
O” unit of the donor molecules were shown to be crucial in asymmetric induction.

 Please wait while we load your content...
Please wait while we load your content...