Tricyclic analogues of epidithiodioxopiperazine alkaloids with promising in vitro and in vivo antitumor activity†
Abstract
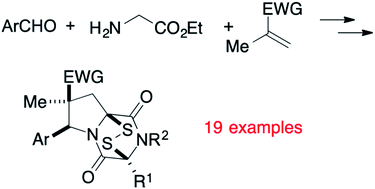
Epipolythiodioxopiperazine (ETP) alkaloids are structurally elaborate alkaloids that show potent antitumor activity. However, their high toxicity and demonstrated interactions with various biological receptors compromises their therapeutic potential. In an effort to mitigate these disadvantages, a short stereocontrolled construction of tricyclic analogues of epidithiodioxopiperazine alkaloids was developed. Evaluation of a small library of such structures against two invasive cancer cell lines defined initial structure–activity relationships (SAR), which identified 1,4-dioxohexahydro-6H-3,8a-epidithiopyrrolo[1,2-a]pyrazine 3c and related structures as particularly promising antitumor agents. ETP alkaloid analogue 3c exhibits low nanomolar activity against both solid and blood tumors in vitro. In addition, 3c significantly suppresses tumor growth in mouse xenograft models of melanoma and lung cancer, without obvious signs of toxicity, following either intraperitoneal (IP) or oral administration. The short synthesis of molecules in this series will enable future mechanistic and translational studies of these structurally novel and highly promising clinical antitumor candidates.

 Please wait while we load your content...
Please wait while we load your content...