Cobalt catalyzed sp3 C–H amination utilizing aryl azides†
Abstract
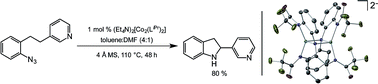
A dinuclear Co(II) complex supported by a modular, tunable redox-active ligand system is capable of selective C–H amination to form indolines from aryl azides in good yields at low (1 mol%) catalyst loading. The reaction is tolerant of medicinally relevant heterocycles, such as pyridine and indole, and can be used to form 5-, 6-, and 7-membered rings. The synthetic versatility obtained using low loadings of an earth abundant transition metal complex represents a significant advance in catalytic C–H amination technology.

 Please wait while we load your content...
Please wait while we load your content...